Empa-PSU team studies mobility of single molecule
Measuring entropy
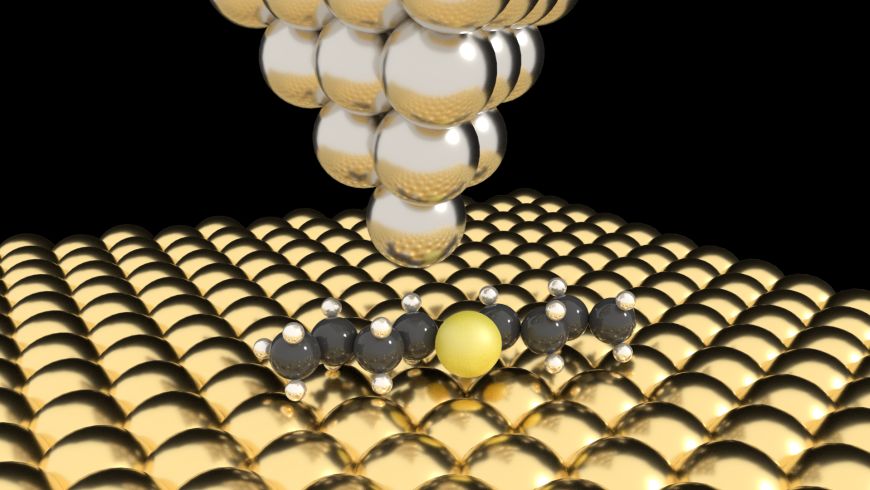
Chemical reactions, especially in biological systems, oftentimes involve macromolecules changing their shape – their “configuration” – for instance, by rotation or translational movements. To study what drives or impedes molecular mobility in more detail chemists and physicists turn to simplified model systems such as individual molecules adhering to a surface. These can then be investigated at temperatures just a few degrees above absolute zero (-273 degrees Celsius) using, for instance, a scanning tunneling microscope (STM), which can probe numerous physical properties of surfaces at the atomic level.
A well-known molecule for this kind of studies is dibutyl sulfide (DBS), a lengthy hydrocarbon with a central Sulphur atom, through which the molecule can be absorbed (attached) to a gold surface. Depending on the temperature the two “arms” rotate more or less easily about the central Sulphur “axis”. There are two physical parameters that are typically used to describe how free to move a molecule on a surface is: the energy barrier it has to overcome to carry out the movement in question – for chemical reactions this barrier is called activation energy –, and the attempt rate, which one can picture as the number of attempts made by the molecule to initiate the movement. And the higher the temperature, the more the two DBS arms rotate (because, at higher temperatures, they are more likely to overcome the energy barrier).
Two weeks of probing a single molecule – with picometer precision
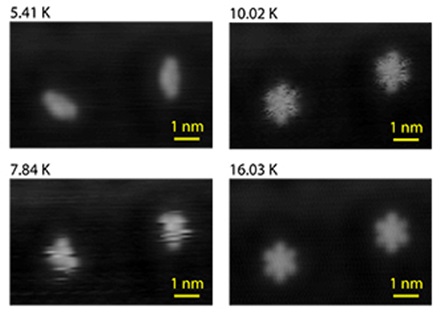
These rotating molecules caught the interest of Empa physicist Hans Josef Hug because their fluctuation rates can be controlled by temperature. This made them an ideal model system for studying non-contact friction and its associated energy losses at the atomic scale. Unfortunately one cannot simply measure non-contact molecular friction with off-the-shelve instruments; but Hug and his team had built a sophisticated low-temperature STM-scanning force microscope (SFM) system capable of operating at temperatures between 4.5 Kelvin and room temperature under ultra-high vacuum conditions with picometer precision. This research attracted Eric Hudson from Pennsylvania State University (PSU) to spend a sabbatical at Hug’s group. As is commonly done in experimental science the Empa-PSU team began by repeating others’ STM work on this molecule.
PhD student Jeffrey Gehrig and postdoctoral researcher Marcos Penedo subjected the DBS molecule to an exhaustive test: over two weeks they mapped the molecule’s rotation rates on a 50 by 50 picometer square grid at eight different temperatures between 5 and 15 Kelvin. When the team evaluated the measured hopping rates to plot the energy barriers for the DBS rotations as a function of the position of STM tip, this “energy landscape” for DBS was not uniform; instead it showed valleys and ridges. In other words: depending on where they positioned the STM tip the DBS arms rotated more or less frequently – at the very same temperature, as the team reports in the recent issue of “Nature Communications”. “That was totally unexpected,” says Hug. “It meant that the tip – which is still relatively far away from the molecule and in no way touches it – somehow influences the molecule’s mobility.”
When nature is revealing its secrets
And not only that: when Gehrig and Penedo plotted the molecule’s attempt rates they obtained a map that looked almost identical to the one with the energy barriers. “This is when I thought: 'Hang on a second, nature is trying to tell us something',” recalls Hug. “Entropy is often thought of as a measure of disorder or randomness, but here it is determined by the number of shapes that the molecule could potentially take, as well as by the number of different ways that the molecule could meet the energy requirements to change its configuration,” explains Eric Hudson, associate professor of physics at PSU. “If the tip of the STM increases the energy required by the molecule to make a change in shape, it is also increasing entropy in the system. In essence, a transition requires a potentially large number of small-energy excitations to co-occur to overcome the energy barrier for a configuration change. The larger the number of excitations required, the more ways in which those excitations may be collected. This multiplicity gives rise to entropy.”
The research team was interested in understanding what drives a molecule’s ability to make changes to its shape — a common requirement of chemical reactions and biological processes., The results of the Empa-PSU team imply that entropy plays a decisive role for the dynamics of the molecule even at very low temperatures where a molecule’s degree of freedom (and thus its “configurational” entropy) is usually significantly reduced and entropy is considered to only play a minor role. “Although entropy is well understood in thermodynamics, it remains more difficult to grasp than other physical quantities”, admits Hug, “perhaps because it is not so much a “property” as a measure of information.” And maybe also because we tend to have a strong association between entropy and chaos – or the “dark side” of entropy –, be it in the kids’ room or on one’s desk.
Imagine dropping a drop of dark blue ink into a glass of water. As time goes by, we will see that the ink mixes with the water until it is homogeneously colored, as if an “invisible force” was at play. Would this process ever reverse on its own and concentrate the ink back into a dark blue droplet? Of course not. Or, as a physicist might put it: “with vanishing probability.” Such everyday experience of what happens spontaneously in isolated systems and what does not is captured by the concept of entropy, which is linked to probability. Thus isolated systems – the ink added to the water glass, say – evolve over time to adopt the most probable of all possible configurations, the one with the highest entropy. Perhaps not surprisingly this is often the most disordered one.
In the case of the rotation of the DBS molecule studied by the Empa-PSU team (see main article) the fascinating observation is that raising the hurdle for the molecule’s rotation – the energy barrier for the movement – simultaneously provides it with a greater number of pathways to overcoming it – hence an increase in entropy. “These findings imply that our home-built STM-SFM becomes a perfect tool for studying a single molecule’s entropy in great detail,” says Empa physicist Miguel A. Marioni.
-
Share
