1. Identification and validation of protein biomarkers
Topical lead: Dr. Peter Nirmalraj
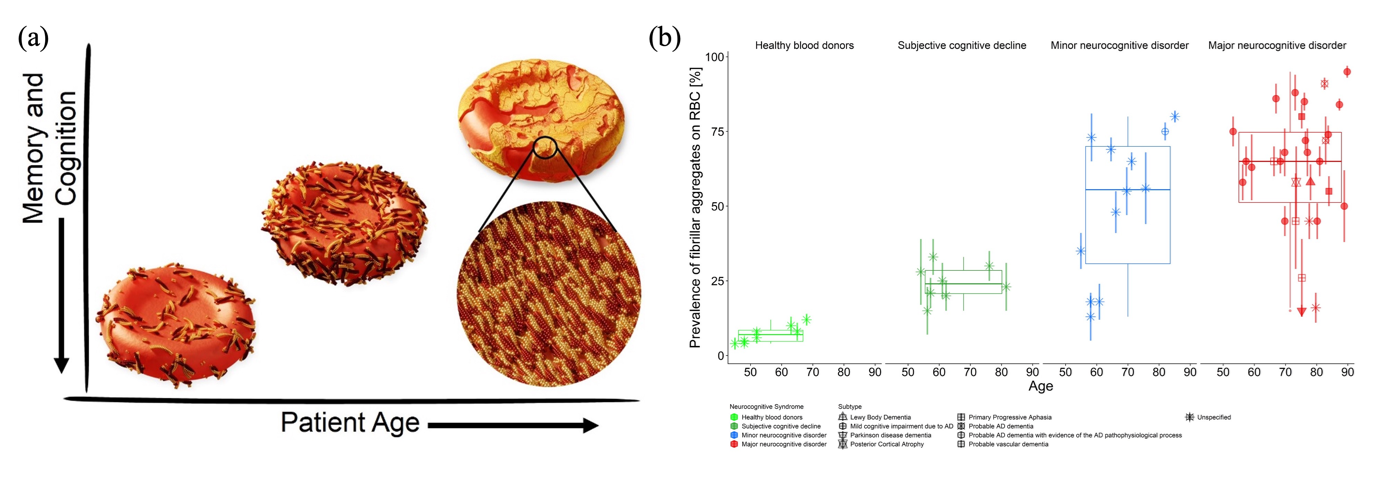
The misfolding and abnormal aggregation of amyloid-β (Aβ) and tau proteins in the brain are the pathological hallmarks of AD. The early identification of individuals with this pathology is crucial for a successful treatment that can slow AD progression, using current behavioural and lifestyle changes as well as future chemo and immunotherapies. Currently, measurements of Aβ (isoforms 1-40 and 1-42, and the 42/40-ratio) and tau protein (total tau and phospho-tau-181 and 217) levels in the cerebrospinal fluid (CSF) and amyloid-PET scans are the gold standards in the clinical detection of AD pathology. Nonetheless, challenges such as the requirement of lumbar puncture decreased levels of Aβ42 in CSF due to their deposition in the brain as plaques and the increased CSF t-tau levels due to other acute disorders remain. In contrast, screening blood plasma for Aβ is less invasive compared to CSF analytics but this medium also hosts numerous other pathological and non-pathological proteins in plasma. Hence, precise quantification is challenging but their diagnostic accuracy is under evaluation as they hold the promise to allow for an inexpensive and minimally invasive method of testing. In general, currently, biomarker-guided methods can quantify the total Aβ40, Aβ42, p-tau, and t-tau in CSF, blood plasma, and serum. However, elemental information on the morphological differences of the pathological protein aggregates in body fluids, which could also shed light on the disease stage, remains largely unavailable to clinicians. To address this unmet need, we at Empa have profiled using an atomic force microscope (AFM) and identified physical biomarkers (differences in size, shape, and prevalence of protein aggregates) as unconventional yet reliable indicators of AD stages (Science Advances, 2021, Brain Communications, 2024).
We aim to further validate the findings in a larger cohort of patients from different demographics and ethnicities using nanoscale imaging and chemical spectroscopy to profile proteins in body fluids.
