Discovery of novel biomarkers for the early stages of chronic wound formation
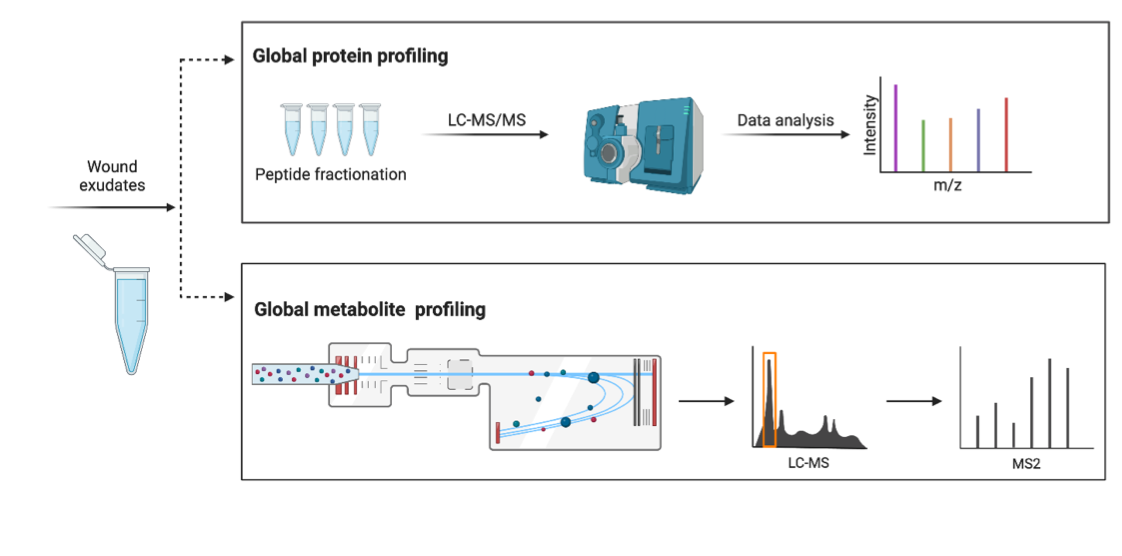
The longer the venous ulcer, diabetic wound or pressure ulcer are present, the higher the risk that the wound will not heal. Therefore, timely identification of instances with a high risk for the chronic wound formation is critical for improving patient care. In this subproject, we will focus on studying venous ulcers, but it is known that chronic wounds of different etiologies share several molecular characteristics and results obtained for one type can be of a broader interest.
Venous ulcers are a common complication of reduced vein blood flow, which can lead to chronic wound development. They affect approximately 1% of population, with the incidence being higher among the elderly (3-4%). Markers, which are commonly used to indicate progression towards chronic wounds - such as pH and temperature, do not provide more specific insights for clinical decision-making and treatment strategies. Moreover, pathology of the underlying molecular processes that lead to the chronic wound development and prevent healthy healing is still incompletely understood. In this work, we will use global mass spectrometry-based proteome and metabolome characterization of wound fluids from venous ulcers which heal within a short time and from those that progress to chronic wounds. The main goal of this work is to identify biomarkers for wounds at a high risk for the progression to a chronic wound, which can be used for informing the design of new sensors. These sensors could be used to monitor wounds in high risk patients in a non-invasive way and could thus be invaluable for supporting early clinical interventions.
Mapping global proteomes and metabolomes of chronic wounds will also enrich our patient-specific knowledge of disease mechanisms for chronic wound formation and possibly even support future development of new therapeutic approaches. In the follow up studies, we will investigate if some of the identified biomarkers could be acting as effector molecules that either promote or suppress wound healing. Therapeutic potential of these molecules will be evaluated using the in vitro models at Empa. If successul, this work can be instrumental for guiding new therapeutic approaches based on the patient-specific molecular makeup and clinical profile.
