A «structured» water layer around cells
Long distance calls by sugar molecules
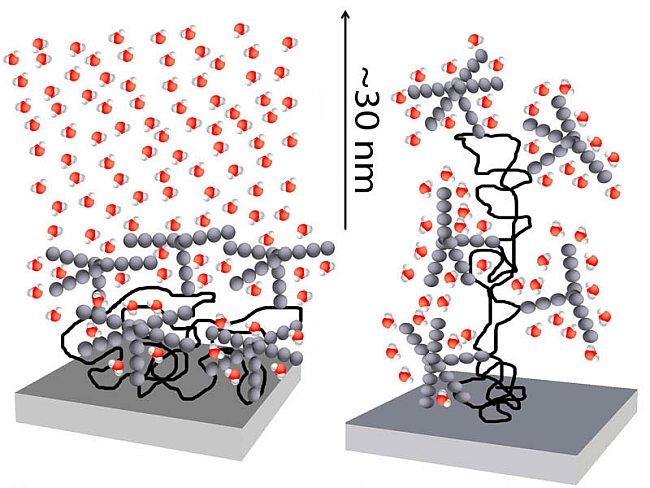
| Caption: A glycoprotein adsorbed on a mica surface in its natural configuration (left) rearranges water molecules across a distance of about 30 nanometers. This effect disappears after unfolding of the glycoprotein (right; source: R. Espinosa-Marzal/ETH Zürich, R. Crockett/Empa). | ||
| Glycoproteins are an essential part of our body: these sugar-protein hybrid molecules are what make the protective mucus that lines our lungs and stomach. They are also part of the fluid that lubricates our joints, the synovial fluid, and cover all our cells, with the sugar parts, the glycans, sticking out like tiny antennae. Researchers in the Laboratory for Surface Science and Technology at ETH Zurich and in Empa’s Laboratory of Nanoscale Materials Science have identified a surprising effect that glycans have on the water molecules that surround them. | ||
| ETH scientist Nicholas Spencer and Empa’s Rowena Crockett along with their colleagues discovered that glycans structure the otherwise random network of water molecules around them. Compared to the size of a water molecule – only about 0.3 nanometers – the distance, over which glycans arrange water molecules, is huge – up to tens of nanometers, i.e. far beyond any expected boundary values. «Since the membranes of our cells are covered in glycans, this may be a way that cells can communicate with each other across water», hypothesizes Spencer. | ||
| Long-range effect on water structure To imitate the configuration glycans acquire on cell surfaces, Rosa Espinosa-Marzal, a co-worker of Spencer, coated a mica surface with a single layer of a well-characterized glycoprotein. While the protein part attached itself to the mica surface, the glycans pointed away from the surface. She then gradually brought a bare mica surface towards the glycoprotein-covered surface, with water between them, and measured the continually increasing repulsive force between them. «To our surprise, there were jumps in this continuous increase in repulsion», explains Espinosa-Marzal, «as if we were squeezing out whole sheets of water and thus relieving the repulsive pressure for a moment.» |
||
| These jumps are caused by the glycans rearranging the network of water molecules above them into clusters or layers. This rearranging effect completely disappeared when the researchers added a chemical to unfold the configuration of the glycoproteins, indicating that the orientation of the glycans is crucial to produce this layering effect on the water molecules above them. | ||
|
This long-range influence of glycans on water may explain why glycoproteins help synovial fluid lubricate our joints so well. Also, the glycan coat that our cells – but also bacteria and fungi – wear can be recognized for instance by our immune system or by receptors on a neighboring cell. By means of their water-clustering properties, glycans may produce a sort of shield around themselves that affects how well a receptor can recognize them. It remains to be seen if cells could indeed communicate across water by means of their glycan coat but it may very well affect the way, in which two cells can sense each other’s presence.
|
||
|
|||
|
|||
|
|||
