Chiral Surfaces
The production of chiral molecules with a single handedness is essential for achieving desired physiological functionality, particularly for pharmaceuticals in medicine. Enantioselective synthesis, which selectively produces one enantiomer more abundantly over the other, requires catalysts with chiral structures or surfaces. Certain intermetallic compounds exhibit such chiral active centers, but their practical application hinges on a comprehensive atomistic understanding of chiral recognition and the mechanisms governing enantioselective reactions.
This research endeavor is dedicated to investigating and comprehending enantioselective on-surface reactions on inherently chiral metallic surfaces from a fundamentally atomistic perspective. Our approach is focused on several key aspects:
- Ultra-high vacuum preparation of atomically well-defined chiral single-crystal metal surfaces
- Examination of chirality transfer from these surfaces to enantioselective adsorption phenomena
- Chirality transfer mechanisms influencing enantiospecific oligomeric coupling reactions
- Chirality transfer dynamics impacting enantiospecific activation (radical formation) in prochiral monomers
Our ultimate objective is to achieve enantiopure on-surface synthesis of chiral products. This research lays the groundwork for the development and practical implementation of chiral intermetallic compounds as heterogeneous enantioselective catalysts.
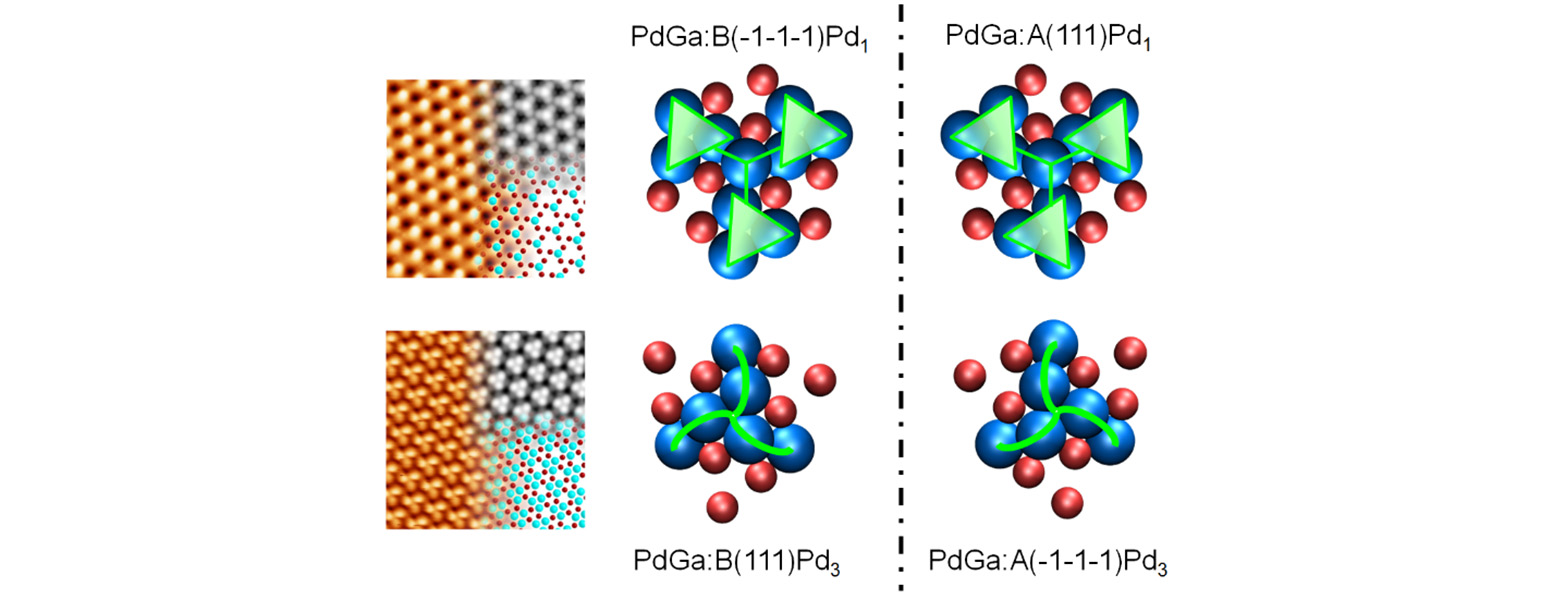
In terms of materials, we currently focus on intermetallic compounds of the P213 space group, in particular stoichiometric PdGa, whose intrinsic chirality makes it a promising candidate for catalyzing enantioselective processes. Pd atoms in this intermetallic compound are spatially separated by Ga atoms, with no segregation occurring below 650°C. We have demonstrated highly enantioselective adsorption of prototypical prochiral molecules on chiral PdGa(111)Pd1 surfaces, achieving enantiomeric excess values close to 1 even at room temperature.
We have also conducted Azide-Alkyne Huisgen Cycloaddition ("click") reactions on chiral PdGa(111) surfaces, with the initial enantiomeric excess of reactants inverted in the products, demonstrating diastereoselective excess and high enantiospecificity.
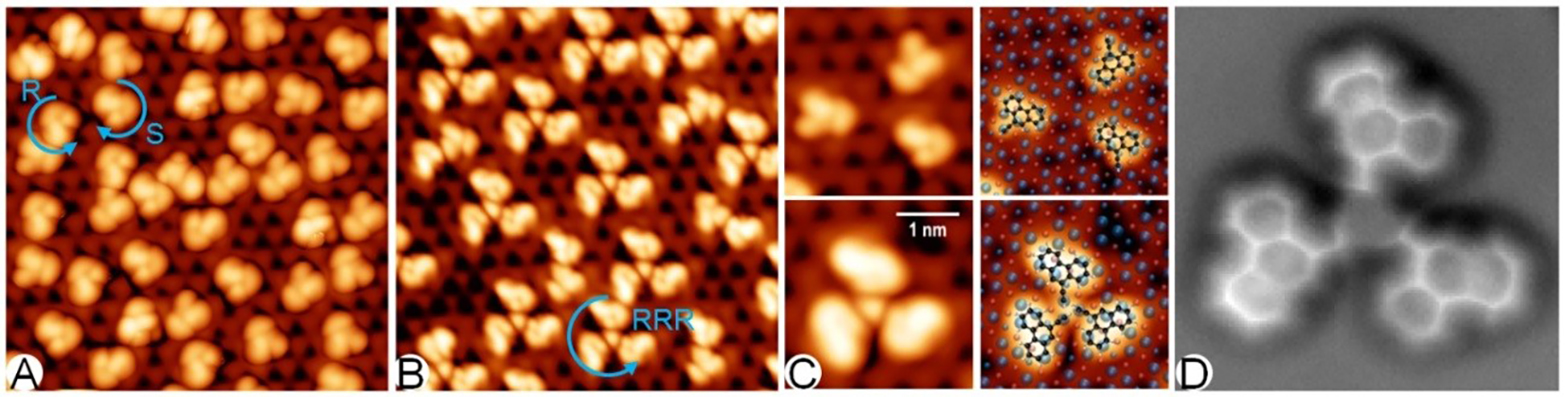
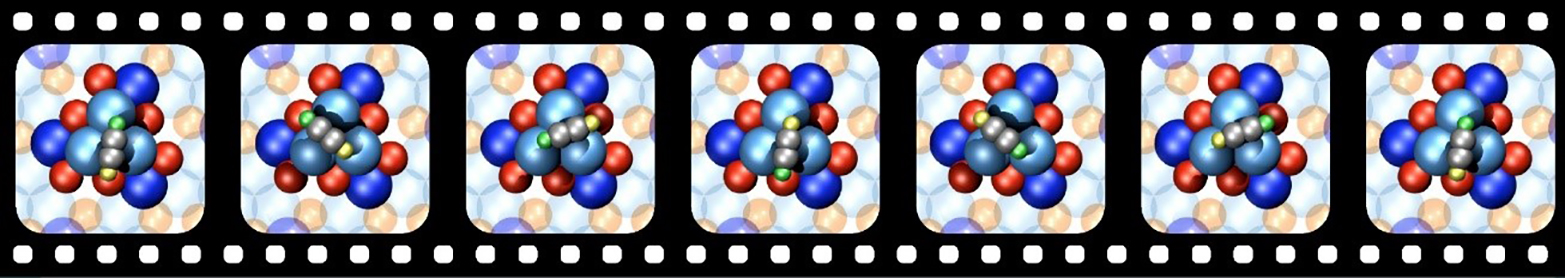
Additionally, we observed chirality transfer in the dynamic behavior of single acetylene molecules adsorbed on Pd trimers, exhibiting highly unidirectional rotation at low temperatures. This world's smallest molecular motor, based on quantum tunneling, raises intriguing questions, e.g. related to the origin of the unidirectional rotation, triggering us to look deeper into the thermodynamic properties of quantum systems.