Catalysis
The presence of the right catalyst can open chemical pathways to high-value products. A catalyst provides the means for reactants to overcome the energy barriers that would otherwise inhibit certain reaction steps. The breaking and forming of new chemical bonds is an atomic scale event, thus to fully understand the workings of a catalyst, one must look at its atomic structure. Catalysts used in heterogeneous catalysis are typically not based on a single material, but a mixture of different ones. In such a system, the interactions between phases are complex and give rise to the active sites that fundamentally govern the catalytic properties of the material. We are interested in studying how the interactions between active phase and support materials are expressed in the catalysts, both ex-situ and in-situ. Aberration-corrected STEM offers a wide range of analytical tools for this purpose, including high-resolution imaging, EDX, EELS, and tomography.
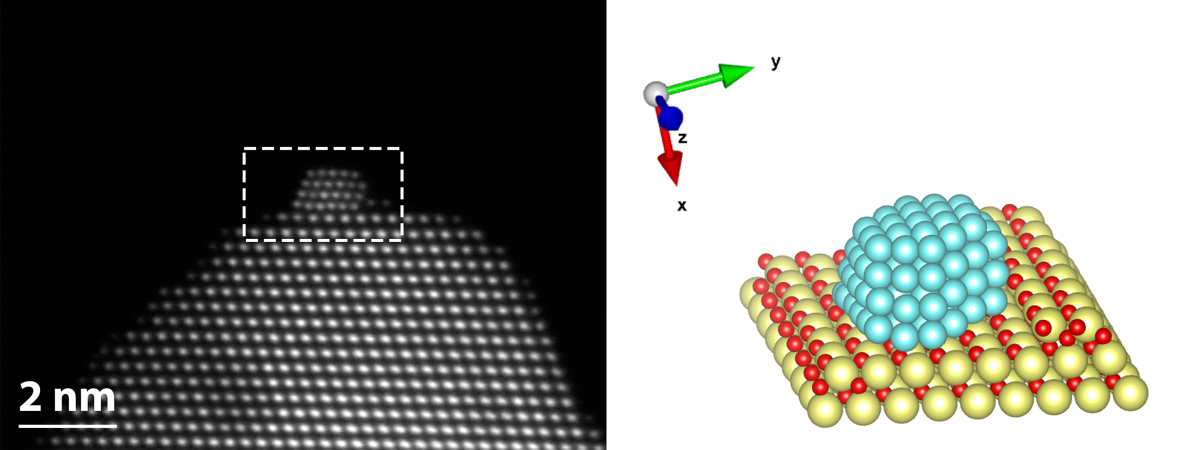
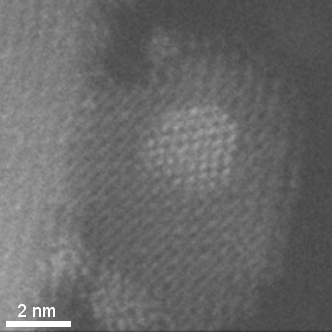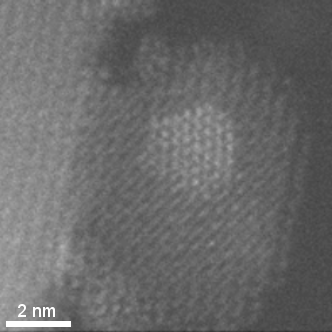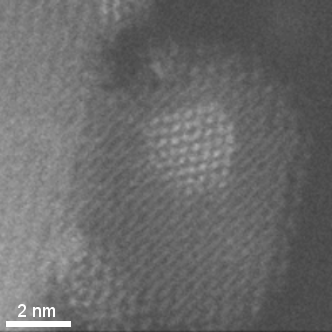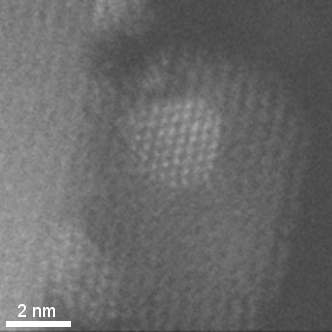

HAADF-STEM tomographic tilt series of platinum particles dispersed on a carbon lace.
-
Share
