Sorption and catalysis at nanosurfaces
Amorphous materials are abundant in nature and can also be generated through thermal treatment of waste, making them promising candidates for sustainable reuse in the context of a circular economy. Due to their disordered atomic structure, such materials exhibit intrinsic physicochemical heterogeneities that can be leveraged to enhance both adsorption and catalytic processes.
At our laboratory, we focus on the multiscale in silico characterization of amorphous substrates for ad-sorption and catalytic applications. We employ atomistic modeling techniques, including Molecular Dy-namics (MD) simulations and Quantum Chemical (QC) calculations—often in combination with enhanced sampling methods—to investigate adsorption phenomena and surface reactions at the nanoscale.
To capture and interpret the structural complexity of amorphous surfaces, we integrate Machine Learn-ing (ML) algorithms. These tools enable us to quantify surface heterogeneities and construct kinetic models based on nanoscale predictions.
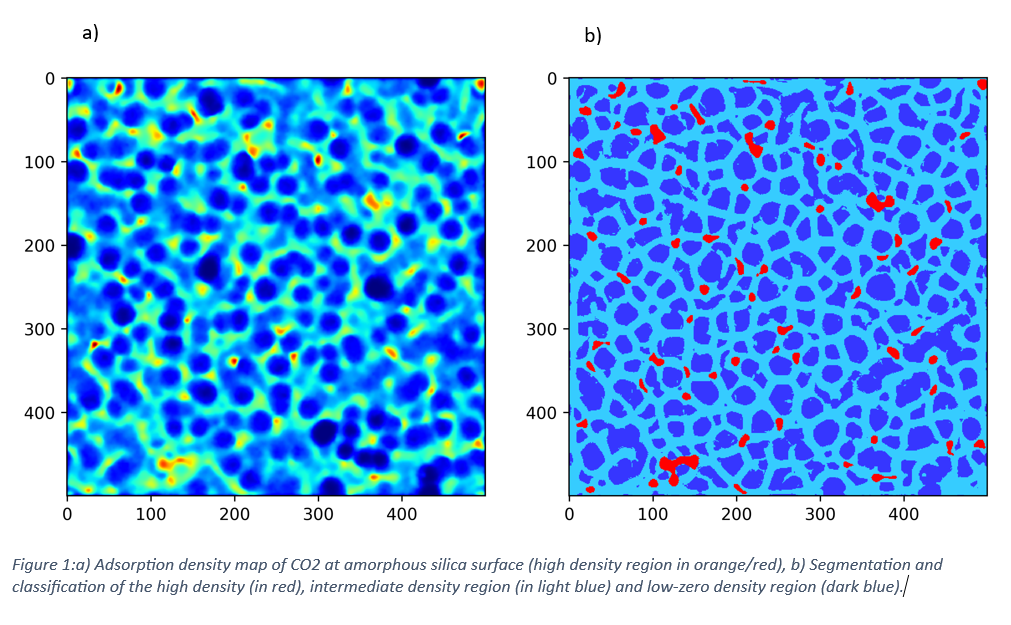
Currently, our research centers on the adsorption of CO₂ at amorphous silica surfaces. We demonstrate how specific features of the surface—such as roughness and coordination defects—promote gas adsorp-tion (Figure 1a). Additionally, ML-based segmentation (Figure 1b) allows us to map and characterize the CO₂ adsorption landscape with high spatial resolution.

