Alkali-aggregate-reaction (AAR)
Two problems arise dealing with alkali-aggregate-reaction (AAR) in structures: how to prevent damages in new structures and how to deal with structures that are already affected? Regarding the first issue, there are different ways in concrete production to prevent deleterious AAR in new structures. One is the use of non-reactive aggregates. However, a concrete producer often has no access to such aggregates either due to regional unavailability or financial reasons. The other one is to reduce the alkalinity of the pore solution in concrete to a level where no deleterious AAR occurs with the aggregate used. This can be achieved by the use of suitable mineral admixtures mitigating AAR or of cement with low Na2O-equivalent.
It has been shown repeatedly that the expansion in mortar or concrete produced with low alkali cement is lower in comparison to high alkali cement. Consequently, various national guidelines and standards take measures to reduce the content of soluble alkalis in concrete by limiting the Na2O-equivalent of the cement or by limiting the cement content. However, the Na2O-equivalent may not always be a reliable parameter defining the potential of cement to cause AAR. Firstly, the alkalis in cement can either be present as potassium and sodium sulfate or be bound in silicate and aluminate phases. Depending on the amount present in the different mineral phases the solubility during the first stage of hydration is different; potassium and sodium sulfate being the more soluble compounds. Secondly, the ratio of potassium to sodium can vary in different cements with consequences for the total amount of K2O and Na2O present. Thirdly, the Na2O-equivalent implies that the effect of potassium and sodium is equivalent. However, there are numerous studies indicating that the influence of potassium and sodium on silica and quartz dissolution is not identical.
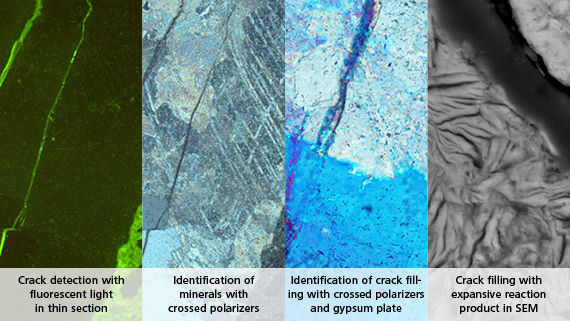
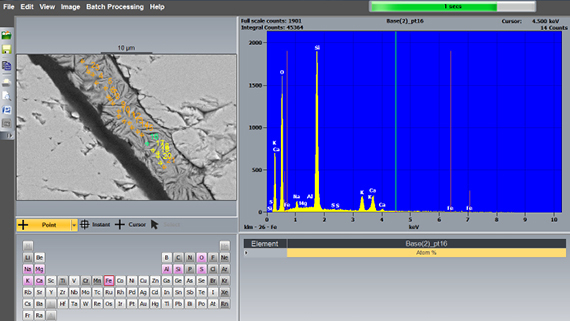

Important publications and documents on AAR
- Leemann, A. (2017). Raman microscopy of alkali-silica reaction (ASR) products formed in concrete. Cement and Concrete Research, 102, 41-47.
- Leemann, A., Katayama, T., Fernandes, I., & Broekmans, M. A. (2016). Types of alkali–aggregate reactions and the products formed. Proceedings of the Institution of Civil Engineers-Construction Materials, 169(3), 128-135.
- Dähn, R., Arakcheeva, A., Schaub, P., Pattison, P., Chapuis, G., Grolimund, D., E. Wieland, Leemann, A. (2016) Application of micro X-ray diffraction to investigate the reaction products formed by the alkali–silica reaction in concrete structures. Cement and Concrete Research, 79, 49-56.
- Leemann, A., Bernard, L., Alahrache, S., Winnefeld, F. (2015). ASR prevention - Effect of aluminum and lithium ions on the reaction products. Cement and Concrete Research, 76, 192-201.
- Leemann, A., Lötscher, L., Bernard, L., Le Saout, G., Lothenbach, B., Espinosa, R. (2014) Mitigation of ASR by the use of LiNO3 - characterization of the reaction products. Cement and Concrete Research, 59, 73-86.
- Leemann, A., Lura, P. (2013) E-modulus of the alkali–silica-reaction product determined by mi-cro-indentation. Construction and Building Materials, 44, 221-227.
- Merz, C., Leemann, A. (2013) Assessment of the residual expansion potential of concrete from structures damaged by AAR. Cement and Concrete Research, 52, 182-189.
- Leemann, A., Merz, C. (2013) An attempt to validate the ultra-accelerated microbar and the concrete performance test with the degree of AAR-induced damage observed in concrete structures. Cement and Concrete Research, 49, 29-37.
- Leemann, A./Lothenbach, B. The influence of potassium-sodium ratio in cement on concrete expansion due to alkali-aggregate reaction. Cement and Concrete Research 2008, 38(10), 1162-1168. /SCI


-
Share
