Hydration
Changes in the solid and liquid phases during hydration
Pore solutions can be collected during the first hours of hydration by vacuum filtration. After hardening, pore solution can be gained using a high pressure device to extract the pore solutions.
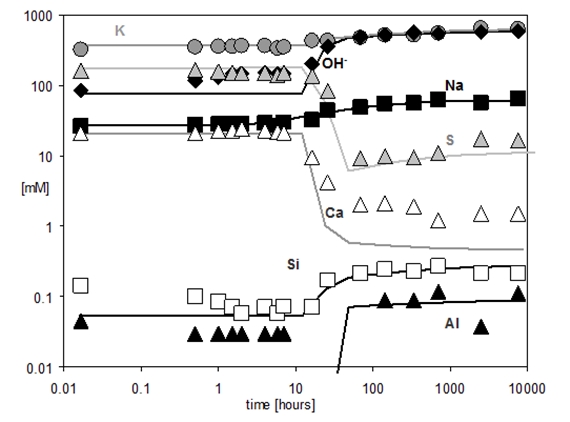
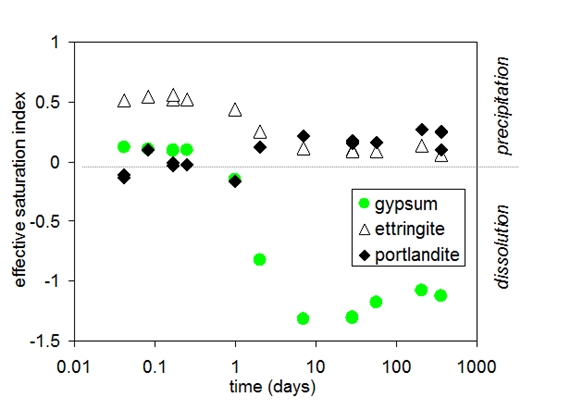
During the first hours the composition of pore solution of Portland cement is dominated by potassium, sodium, sulfate, hydroxide and calcium. A significant change in the composition of the pore solution is observed between 6 and 24 hours; calcium and sulfate concentrations decrease as solid gypsum and anhydrite are depleted due to the formation of ettringite, while hydroxide concentrations increase at the same time.
Important publications
In the last years we have carried out hydration studies in pure Portland cement systems, in blended systems, in calcium sulfoaluminate cements, in alkali activated slag and fly ash and supersulfated slag systems.
- Xu, B., Lothenbach, B., Leemann, A., Winnefeld, F. (2018) Reaction mechanism of magnesium potassium phosphate cement with high magnesia-to-phosphate ratio, Cement and Concrete Research, 108, 140-151
- Le Saout, G., Lothenbach, B., Taquet, P., Fryda, H. and Winnefeld, F. (2018) Hydration of calcium aluminate cement blended with anhydrite, Advances in Cement Research, 30, 24-36.
- Vollpracht, A., Lothenbach, B., Snellings, R., Haufe, J. (2016) The pore solution of blended cements: a review. Materials and Structures, 49(8), 3341-3367
- Lothenbach, B./Le Saout, G./Ben Haha, M./Figi, R./Wieland, E. Hydration of a low-alkali CEM III/B–SiO2 cement (LAC). Cement and Concrete Research 2012, 42(2), 410-423.
- Schöler, A., Lothenbach, B., Winnefeld, F., Zajac, M. (2015) Hydrate formation in quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cement and Concrete Composites, 55, 374-382.
- Deschner, F., Lothenbach, B., Winnefeld, F. (2013) Effect of temperature on the hydration Portland cement blended with siliceous fly ash. Cement and Concrete Research, 52, 169-181.
- Ben Haha, M./Le Saoût, G./Winnefeld, F./Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cement and Concrete Research 2011, 41(3), 301-310.
- De Weerdt, K./Ben Haha, M./Le Saoût, G./Kjellsen, K. O./Justnes, H./Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cement and Concrete Research 2011, 41(3), 279-291.
- Gruskovnjak, A., Lothenbach, B., Winnefeld, F., Figi, R., Ko, S.-C., Adler, M., Mäder, U. (2008) Hydration mechanisms of super sulphated slag cement. Cement and Concrete Research, 38(7), 983-992.
- Lothenbach, B., Winnefeld, F., Alder, C., Wieland, E., Lunk, P. (2007) Effects of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cement and Concrete Research, 37(4), 483-491.
- Lothenbach, B. and F. Winnefeld (2006) Thermodynamic modelling of the hydration of Portland cement. Cement and Concrete Research 36, 209-226.

Prof. PD. Dr. Barbara Lothenbach
Senior Researcher / Projektleiterin / Adjunct Prof. NTNU

