Thermodynamic modeling
Thermodynamic modeling of hydration
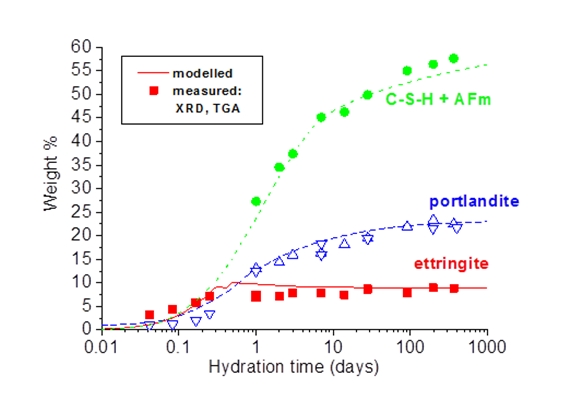
The hydration of cements can be assumed to take place via dissolution and precipitation processes. Coupling of thermodynamic modelling with a set of kinetic equations which described the dissolution of the clinker, can predict quantitatively the amount of hydrates as a function of the time and temperature of hydration: A set of empirical expressions is used to estimate the degree of dissolution of each clinker mineral as a function of time. Based on the composition of the cement and thermodynamic modelling the quantities of and volumes of hydrates form can be calculated. The quantities of ettringite, portlandite and amorphous phase as determined by TGA and XRD agree generally well with the calculated amounts of these phases after different periods of time. The findings as documented in different papers show that changes in the bulk composition of hydrating cements can be followed by coupled hydration - thermodynamic models. Comparison between experimental and modelled data helps to understand in more detail the dominating processes during cement hydration.
This approach has been successfully applied to study the hydration of Portland cements, alkali activated slags, supersulfated slags, calcium aluminate calcium aluminate cements and calcium sulfoaluminate cements as well as to blended cements containing limestone, fly ash, slag, or metakaolin.
Important publications
- Martin, L., Winnefeld, F., Tschopp, E., Müller, C., Lothenbach, B. (2017) Influence of fly ash on the hydration of calcium sulfoaluminate cement, Cement and Concrete Research 95, 152-163.
- Winnefeld, F., Lothenbach, B. (2016) Phase equilibria in the system Ca4Al6O12SO4 – Ca2SiO4 – CaSO4 – H2O referring to the hydration of calcium sulfoaluminate cements, RILEM Technical Letters, 1, 10-16.
- Lothenbach, B., Rentsch, D., Wieland, E. (2014) Hydration of a silica fume blended shotcrete cement. Journal of Physics and Chemistry of the Earth, 70-71, 3-16.
- Zajac, M., Rossberg, A., Le Saoût, G., Lothenbach, B. (2014) Influence of limestone and anhydrite on the hydration of Portland cements, Cement and Concrete Composites, 46, 99-108.
- Deschner, F., Winnefeld, F., Lothenbach, B., Dittrich, S., Goetz-Neunhoeffer, F., Neubauer, J. (2012) Hydration of Portland cement with high replacement by siliceous fly ash. Cement and Concrete Research, 42 (10), 1389-1400.
- Lothenbach, B./Le Saout, G./Ben Haha, M./Figi, R./Wieland, E. Hydration of a low-alkali CEM III/B–SiO2 cement (LAC). Cement and Concrete Research 2012, 42(2), 410-423.
- Ben Haha, M./Lothenbach, B./Le Saoût, G./Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag - Part II: Effect of Al2O3. Cement and Concrete Research 2012, 42(1), 74-83.
- Ben Haha, M./Lothenbach, B./Le Saoût, G./Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag - Part I: effect of MgO. Cement and Concrete Research 2011, 41(9), 955-963.
- Winnefeld, F./Lothenbach, B. Hydration of calcium sulfoaluminate cements: experimental findings and thermodynamic modelling. Cement and Concrete Research 2010, 40(8), 1239-1247.
- Lothenbach, B., Le Saout, G., Gallucci, E., Scrivener, K. (2008) Influence of limestone on the hydration of Portland cements. Cement and Concrete Research, 38(6), 1162-1186.
- Lothenbach, B., Matschei, T., Möschner, G., Glasser, F. (2008) Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cement and Concrete Research, 38(1), 1-18.

Prof. PD. Dr. Barbara Lothenbach
Senior Researcher / Projektleiterin / Adjunct Prof. NTNU

-
Share
