Determination of Thermodynamic Data and Chemical Structure for Zeolites
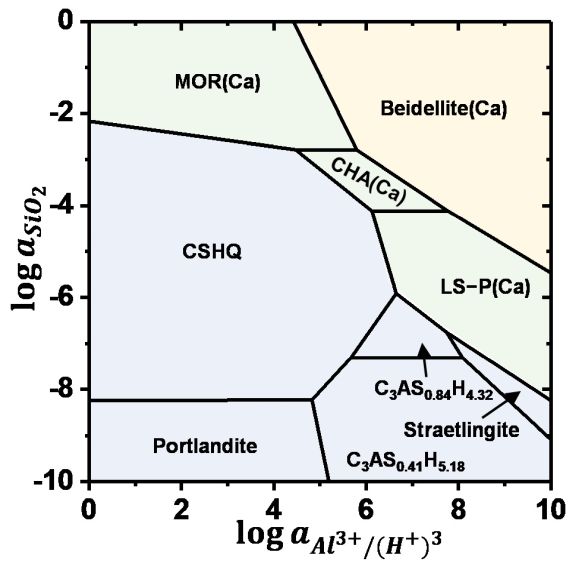
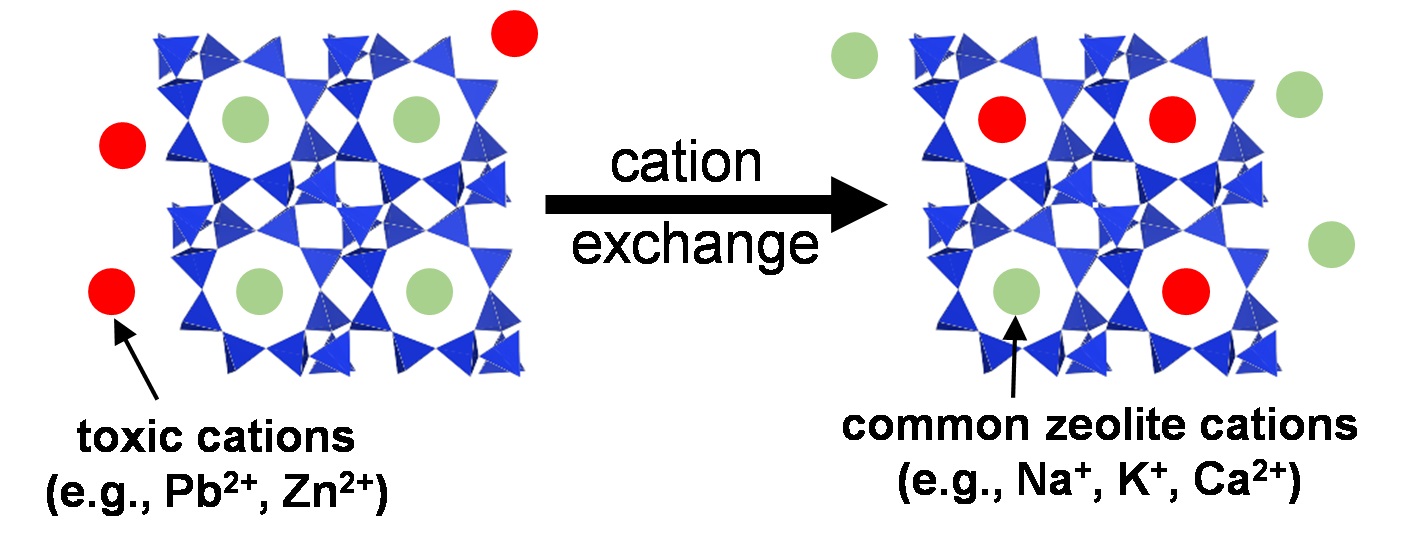
Zeolites are widely used in areas as diverse as petrochemical industries, agriculture, and as supplementary cementitious materials. Zeolitic precursors are also formed in the interaction zone between cement and clay rocks, in alkali-activated cements, at the surface of cements exposed to seawater and during carbonation. Due to their high structural variability in terms of the Al/Si and extra-framework cations, their stability is poorly known, which leaves a serious gap in thermodynamic data and thus disables reliable predictions on their durability at the interaction zone of cement with the environment. The solubility of zeolites with different Al/Si ratios and cations (Na, K, and Ca) are measured at different temperatures. The experimentally derived solubility data allow predicting under which conditions zeolite might forms, facilitating understanding the formation conditions. The binding of Na+, K+, Al3+ and/or heavy metal in zeolites can contribute to counteract and prevent the expansive alkali silica reaction or to slow down the transport of heavy metals. Prof. Dr. Barbara Lothenbach and Dr. Bin Ma are investigating this topic in the Group Cement Chemistry and Thermodynamics.
Project Period
April 2018 - July 2021
Additional information
Funding Organization
Empa (Distinguished Senior Researcher grant)
EU Horizon 2020 (Marie Skłodowska-Curie grant)
Nagra
Synthesis, characterization, and thermodynamic study of selected Na-based zeolites
Thermodynamic study of cement/rock interactions using experimentally generated solubility data of zeolites
-
Share
