Reactive Gasphase Flame Inhibition Species
Owing to the lack of suitable analytical techniques for selective and instantaneous analysis of the transient species formed in the thermal decomposition of OPCs, the elucidation of the gas-phase activity of phosphorous compounds is quite challenging. Important questions are still open, such as how individual bond energies in the flame-retardant molecules determine their fragmentation into gas-phase active products under thermal stress, what intermediates are formed under heat, and how flame-retardant efficiency can be maximized by targeted incorporation of specific functional groups into the flame retardant.
Our main objective is to identify the intermediates and the routes that generate the key phosphoryl radicals in the gas-phase flame retardant action, with a special emphasis on the POX generation capabilities of OPCs. In our research we have chosen dimethyl methyl phosphonate (DMMP) as a model OPC and studied the thermolysis mechanism using a unique experimental setup (pyrolysis – VUV photoionization-iPEPICO) 1. Identification of the product distribution as a function of pyrolysis temperature contributes to our understanding of fire suppression and helps us in proposing FR candidates with enhanced POx release capabilities. Different computational chemistry approaches are applied in this study to augment the experimental data, including ab initio calculations to map the potential energy surface of the neutral state, and locate transition states and minima to verify and validate the proposed decomposition mechanisms.
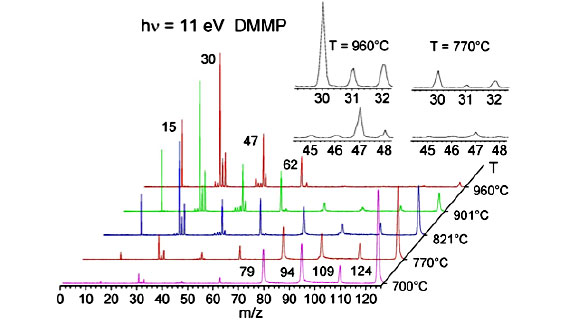
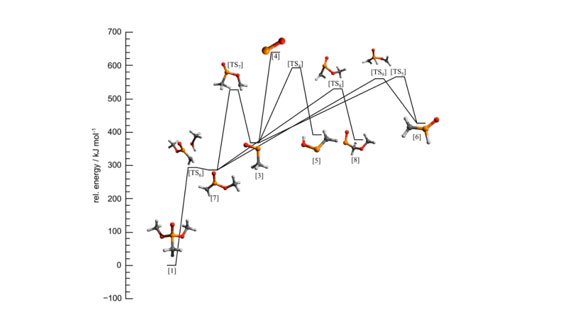
The above figure shows one of the pathways of the proposed decomposition of DMMP. This unimolecular decomposition pathway of DMMP shows formation of PO*, which is considered as an important flame inhibition. Formation of other HxPOy species requires oxidative conditions.
Reactive species such as ∙PO2 and HOPO are considered of upmost importance in flame inhibition and catalytic combustion processes of fuels. However, the underlying chemistry of their formation remains speculative due to the unavailability of suitable analytical techniques which can identify the transient species that lead to their formation. This study elucidates the reaction mechanisms of formation of phosphoryl species from dimethyl methyl phosphonate (DMMP) and dimethyl phosphoramidate (DMPR) under well-defined oxidative conditions. It was found that ·PO2 formation requires oxidative conditions above 1070 K. The combined presence of O2 and H2 led to significant changes in the decomposition chemistry of both model phosphorus compounds leading to the formation of ·PO2. The reaction ·PO + O2 → ·PO2 + ·O· was identified as the key step in the formation of ·PO2. The findings of this study shed light onto decomposition pathways of organophosphorus compounds, which are beneficial for their fuel additive and fire suppressant applications 2.
1. Liang, S., et al., Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, a Key Species in Flame-Retardant Mechanisms, in Chemistry – A European Journal. 2015, WILEY-VCH Verlag. p. 1073-1080. (https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201404271)
2.'The Underlying Chemistry to Formation of Phosphoryl Radicals from Organophosphorus Compounds - A Missing Puzzle Piece in the Flame Chemistry' : Authors: Shuyu Liang, Patrick Hemberger, Mathias Steglich, Pietro Simonetti, Joëlle Levalois-Grützmacher, Hansjörg Grützmacher, and Sabyasachi Gaan; Chem. Eur. J. 10.1002/chem.202001388 (https://doi.org/10.1002/chem.202001388)

-
Share
