Fluid-Solid Interphase
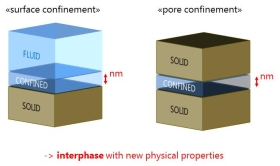
Fluids next to solid surfaces or adjacent to solids have physical properties that differ from those found in the bulk phase. These new physical properties of this nanometer thick interphase are in the focus of this research.
The term “confinement” is often used to describe this nanometer thick interphase of fluid with new physical properties, which occurs in intensified form in a nanometer gap between two solids.
But what are these new physical properties? Let us see some examples:
Understanding oleophobicity in the con-text of PFAS removal and replacement
Per- and polyfluoroalkyl substances (PFAS) are organic fluorinated molecules used across numerous industries for their properties, namely water- and oil-repellency, and heat- and soil-resistance. One of the reasons for current difficulties in developing alternatives is oil-repellency (oleophobicity), which is not well understood. We study model systems to improve our understanding of oleophobicity and to inform how we improve PFAS detection and removal methods.
A. The extended surface force apparatus (eSFA).
B. The lenses used to support the interferometric stack onto which the plasma polymer film is deposited. The interac-tions between these two surfaces are measured with high precision.
C. Graph repre-senting the decay of the electric potential from the surface (not to scale). The eSFA allows us to probe this region in the liquid.
Oleophobicity in the context of PFAS removal and replacement
PFAS and oleophobicity
Per- and polyfluoroalkyl substances (PFAS) are fluorinated organic molecules heavily used in industry and in daily life for their interesting properties. PFAS display water- and oil-repellency (also known as hydrophobicity and oleophobicity), as well as soil- and heat-resistance, thanks to the presence of the carbon-fluorine bond. Well-known everyday examples of PFAS include Teflon (used for cooking ware) and Gore-tex (used for textiles).
Unfortunately, PFAS are also harmful to the environment and to living beings; indeed, the starting materials and degradation products of PFAS are known to be persistent and toxic. It has become clear that it is necessary to find alternatives, but as of yet, there are no competitive substitutes available to replace many essential PFAS, which illustrates the need for a more fundamental understanding of how PFAS and their potential substitutes interact with liquids.
Current understanding of oleophobicity and the physico-chemical factors that control it is somewhat limited. Some factors, such as the influence of the C-F bond and topography of the substrate, have been established as important, but not fully quantified. Other factors, such as the role of near surface forces (changes in the Gibbs free energy of the liquid near the surface) or dissolved gases and water are not fully understood.
In order to efficiently and appropriately substitute PFAS, it is essential to gain better understanding of how these substrates interact with water and oils, and to understand how best to mimic these properties without resorting to the used of the C-F bond. This understanding is also critical to improving the detection and removal of PFAS already present in the environment, particularly in water.
Ongoing work
In the Surfaces and Interfaces Laboratory, we are studying how liquids behave at PFAS model surfaces and at fluorine-free model surfaces made via plasma polymerization. Thin films are deposited onto a substrate by introducing a monomer gas into a plasma chamber, where it is broken into reactive species that react with each other and form a polymer on the surface of the substrate. These plasma polymer films are both suitable for industrial applications and for fundamental studies.
By deepening our understanding of how molecules of water and oils interact with these thin plasma polymer films, we can gain knowledge on how PFAS interact with aqueous and oily environments, as well as how fluorine-free substitutes perform in comparison.
Fluorine-free model surfaces allow us to understand how potential PFAS substitutes perform in different environments and provide clues as to what improvements can be made. PFAS model surfaces help us understand how oils behave near such surfaces compared to fluorine-free surfaces.
Ongoing work is focused on understanding the oleophobicity displayed by PFAS and whether the fluorine-free plasma polymer films, which are not oleophobic, can be used to capture PFAS fragments in aqueous environments, either for detection or removal purposes. Studying how fluoroalkanes interact with fluorine-free model systems will inform us on how PFAS fragments interact with the environment.
Modified water structure at the interphase
Water is indisputably the most relevant small molecule in the biosphere. It is involved in biochemical reactions inside our cells as well as on the surface of implants or technical surfaces that are exposed to humid atmospheric influence.
The water molecule is known to exhibit an unusually complex behavior due to the different molecular interactions that are possible with a water molecule; these include Van der Waals interactions, dipolar interactions as well as hydrogen bonding, which can affect the orientation of the molecules. In part of our research we want to shift the relative strength of these interactions by a modification of the local environment in the vicinity of water molecules. One implementation is to confine water inside a matrix where the water-water distance is increased, so that hydrogen bonding is effectively switched off while the longer-ranged dipolar interaction remains intact. A shift of interaction strengths in the opposite direction is possible by implantation of a dipole moment at the confining surface. We study the effect of such measures on the water structure at the interphase using a unique version of the extended Surface Forces Apparatus that allows full atmospheric control. This means we can set and maintain conditions that are commonly not attainable, like degassed water, gas-enriched solvents, supercritical CO2 and other interesting loci of the (fluid bulk) phase diagram…
References:
- D. Hegemann, N. Hocquard and M. Heuberger, Scientific Reports 2017, 7.
The inner structure of electrical double layers
Another important example of an interphase is given if the fluid contains ions (e.g. salt solution). Here, the solid surface typically acquires a net electrical charge and counter ions from the salt solution form a so-called electrical double layer (EDL). While this phenomenon is long known and used in technological applications, the theoretical understanding of the inner structure is far less clear. For instance, the well-established DLVO theory breaks down in very narrow pores or at salt concentrations as low as 10% of saturation. We can study the structure of such EDLs using the extended surface forces apparatus (eSFA) in collaboration with the Laboratory for Surface Science and Technology at the ETH Zürich, the Paul Scherrer Institute and the University of Illinois, USA. We find that the salt (counter) ions carry along a hydration shell, forming some a “soft ball” of a few Ångstom size that can layer next to the surface, like tennis balls on the floor of a box. We can sense these layers of balls using the high force sensitivity of the instrument and even detect sub-atomic transitions, which are related to the change of hydration water structure in the system. Knowing better the inner structure of EDLs is important for biological interaction and also technically relevant fields like rock weathering.
References:
- M. P. Heuberger, Z. Zachariah, N. D. Spencer and R. M. Espinosa-Marzal, Physical Chemistry Chemical Physics 2017, 19, 13462-13468.
- Z. Zachariah, R. M. Espinosa-Marzal and M. Heuberger, Journal of Colloid and Interface Science 2017, 506, 263-270.
Critical and supercritical Casimir forces
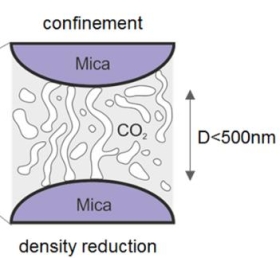
Our unique pressurized surface forces apparatus can also be used to study critical and supercritical fluids like CO2 under confinement. The new physical insights gained there are potentially important for future technological developments in the area of polymer processing and polymer foaming. In particular, we could show that order parameter (=density) fluctuations near the critical point prevail unexpectedly and measurably along the extension of the CO2 coexistence line and lead to strong attractive forces in near critical and surprisingly also into supercritical conditions. This force is expected to have a pore stabilizing effect, for example, during pore formation in polymers processed with critical and supercritical fluids.
References:
- Schurtenberger, E. and M. Heuberger (2011). "The extended surface forces apparatus. Part IV: Precision Static Pressure Control." Review of Scientific Instruments 82(103902): 8.
- Schurtenberger, E. and M. Heuberger (2012). "Supercritical Casimir Effect in Carbon Dioxide." Journal of Supercritical Fluids 71: 120-126.
Adsorption Sensing

In parallel to measuring surface forces and molecular interactions we are also using a transmission interferometric adsorption sensor (TInAS) to assess the adsorption of molecules onto surfaces. The method is sensitive enough to detect the adsorption isotherm of water on mica crystal surface or the coupling of antigens with surface immobilized antibodies. More recently we use this method to study the properties of different surface functionalization’s and novel plasma polymer films, for example, in terms of protein adsorption.
We also work on extending this method now to be able to detect changes in refractive index of an molecularly adsorbed film; this would allow us to follow solvation and conformational changes of adsorbed molecules. Besides offering a comparable sensitivity with other bio-sensing methods, the TInAS methode is significantly more simple and less expensive.
References:
- Balmer, T. E., et al. (2008). "The Effect of Surface Ions on Water Adsorption to Mica." Langmuir 24: 1566-1569.
- Heuberger, M. and T. Balmer (2007). "The Transmission Interferometric Adsorption Sensor." Journal of Physics D: Applied Physics 40: 7245-7254.
- Sannomiya, T., et al. (2010). "Optical Sensing and Determination of Complex Refelection Coefficients of Plasmonic Structures using Transmission Interferometric Plasmonic Sensor." Review of Scientific Instruments 81: 053102.


