Bacteria and Materials Interactions
The Bacteria & Materials Interaction Group focuses on understanding, predicting and controlling material-bacteria interactions for health-related applications. In particular, we combine interdisciplinary knowledge in the pursuit of understanding how bacteria adhere to surfaces, and how the adhesion can be controlled. Among our core strengths are the expertise ranging from microbiology to biomaterials, and the long-term experience in working with hospitals, academic partner institutions, and industry.
Our key research aspects include:
- Studying mechanisms of bacterial adhesion on surfaces, particularly bacterial mechanobiology, aiming at antibacterial and anti-adhesive surfaces
- Studying mechanisms of biofilm resistance toward antimicrobials
- Development of biosensors for detection of antimicrobial resistant bacteria
- Design of novel approaches for treatment of antimicrobial resistance
Research Activities
Bacteria-Materials interactions
Upon sensing and interacting with materials, bacteria trigger a variety of changes in gene expression, including the genes essential for cell-cell communication, motility and surface attachment. Although changes in these phenotypes have been observed, the mechanisms used by bacterial cells for sensing and responding to surfaces are not well understood. To develop novel classes of materials that inhibit or reduce bacterial adhesion or proliferation, a better understanding of these mechanisms is necessary. Our group is interested in studying the underlining mechanisms of surface sensing at the molecular level and the consequent impact on cell adhesion.
We have close collaborations with Dr. Dominik Abt (Cantonal Hospital St. Gallen), Prof. Dr. Leo Eberl (University of Zurich), Prof. Dr. Henny van der Mei (University Medical Center Groningen) and Prof. Dr. Songmei Wu (Beijing Jiaotong University). We greatly acknowledge support from Innosuisse.
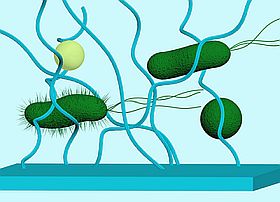
The physicochemical properties of a material surface impact bacterial adhesion, which consequent can trigger altered gene expression.
Antimicrobial resistance (AMR) of biofilm
Infections are often caused by bacterial biofilms. Bacteria living in biofilms can tolerate much higher antibiotic concentrations compared to planktonic bacteria and survive long enough to evolve antimicrobial resistance (AMR). Our group aims to investigate how bacteria generate resistant during biofilm formation on medical devices, and utilize the gained knowledge to develop novel antimicrobial strategies to prevent biofilm-associated infection and AMR.
We have close collaboration with Dr. Baharak Babouee Flury (Cantonal Hospital St. Gallen), Dr. Frank Schreiber (BAM), Prof. Dr. Henny van der Mei (University Medical Center Groningen), Prof. Jeremy Webb (University of Southampton), and Dr. Christian Ahrens (Agroscope). We greatly acknowledge the support from the Swiss National Science Foundation on our project "Partnership against biofilm-associated expression, acquisition and transmission of AMR".
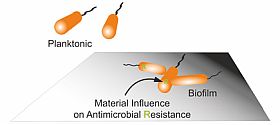
Understanding mechanism of biofilm resistance toward antimicrobial materials by identifying involved genes and pathways.
Biosensors for detection of antimicrobial resistant bacteria
We combine material science, chemistry, and biology to design non-invasive biosensors highly specific for resistant bacteria. The biosensors, including those for evaluation of skin wound infections, dental diseases, sepsis and pneumonia etiology, have potential to allow surgeons to achieve rapid diagnosis and initiate appropriate treatment timely.
Diverse projects are carried out in close collaboration with Prof. Dr. Inge Herrmann (ETHZ/Empa), Prof. Dr. Simone Schurle (ETHZ), Dr. Saso Jezernik and Dr. Christian Jäggi (Bioinitials), Dr. Silvia Generelli (CSEM), Dr. Stefan Stübinger (University of Basel), Dr. Werner Albrich and Dr. Christian Kahlert (Cantonal Hospital St. Gallen). We greatly acknowledge support from Innosuisse.
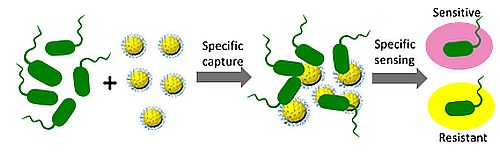
Novel approaches for treatment of antimicrobial resistance
To reduce development of antimicrobial resistance, we utilize natural materials, hazard-free and easily accessible approaches to replace antibiotics to treat biofilm associated chronic wounds. These bioinspired wound dressings not only allow elimination of biofilms but also promote tissue re-epithelization and wound healing.
This project is carried out in close collaboration with Dr. Zhihao Li (University Hospital Zürich), Prof. Jörg Grünert (Canton Hospital St. Gallen) Prof. Sven Panke and Dr. Martin Held (ETHZ). We greatly acknowledge the support from the Swiss National Science Foundation on our project "Probiotics for treatment of skin wound infection: fighting biofilms and promoting tissue re-generation" starting on June 1st, 2021.
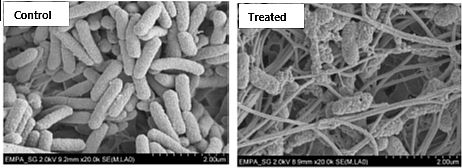
Biofilm Lab and characterization tools
Our group hosts a Biosafety Class II Lab where the group works with different bacterial strains including Gram-negative and Gram-positive species and multi-resistant pathogens. We are also equipped with a dedicated lab for co-culture of bacteria and mammalian cells. We study biofilms in microtiter plates, flow chambers, as well as microfluidic systems under static or dynamic conditions. Bacterial adhesion and biofilms on surfaces are analyzed by different methods such as Crystal violet assays, Fluorescence assays, Microscopy (Wide-field, CLSM, SEM), Real-time quantitative PCR.
Dr. Clara Guarch Perez
PostDoc
Flavia Zuber
Laboratory technician
Microbiology, in-vitro biofilm assays, cell culture, cytotoxicity testing
Stefanie Altenried
Laboratory technician
In vitro biofilm formation and assessment, Microbiology, Biotechnology, molecule biology
Sixuan Zhang
Liubov Shishaeva
PhD candidate
Siyuan Tao
PhD candidate
Anna Hu
PhD candidate
Maria Laura Zonta
Guest Master student
Fathima Moidu
Guest Master student
Phu Binh Nguyen
Guest Master student



