Magnesium orthopaedic screws
The Screw That Dissolves
Where bones fracture, surgeons often have to join the fragments with implants. Magnesium orthopaedic screws, which over time dissolve in the body, spare patients another operation after healing is completed and reduce the risk of infection. What happens inside the body during this process, though, is still largely unknown. To develop optimized alloys and orthopaedic screws with functionalized surfaces, Empa researchers are now investigating magnesium corrosion.
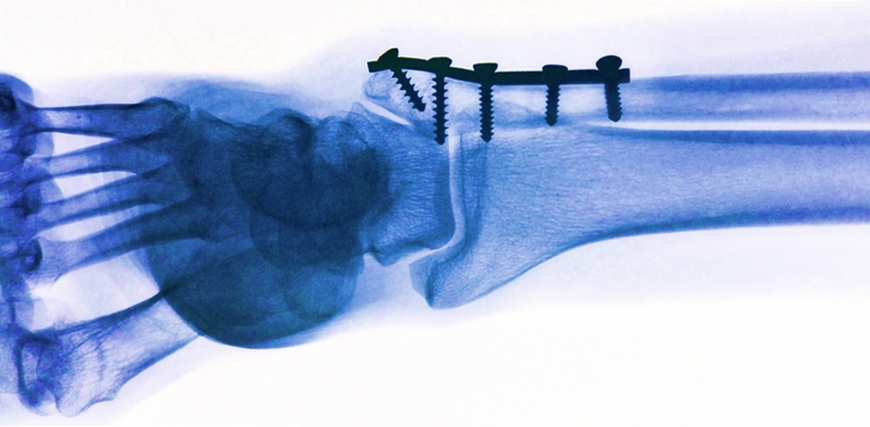
When surgeons want to fix bone fragments after a fracture, the critical question is what type of implants to use: screws and plates made of titanium or steel, which are mechanically and chemically very stable in the body, but have to be removed later on by another surgical procedure? Or implants made of organic materials that dissolve over time but can have certain other drawbacks, such as lack of mechanical strength or unfavourable degradation products? Empa researchers are currently working on solving this dilemma: tiny magnesium implants and screws. These are mechanically robust at first but dissolve later on in the body in a controlled way that does not cause tissue damage.
Such magnesium implants are particularly interesting for medical orthopaedic applications in children whose bones are growing rapidly. The biodegradable screws do not impair the child's bone growth and save the small patients a second surgery. In addition, the risks of infection can be minimized and costs can be cut. "Magnesium is more commonly regarded as a white powder that is often taken as a dietary supplement," says Arie Bruinink from Empa's Laboratory for "Joining Technologies and Corrosion". Implants made of magnesium alloys are not only biocompatible, however; they also have mechanical properties during the first delicate healing phase that are bonelike and, therefore, even more suitable than those of titanium.

The blessing of a resorbable screw can also be its curse. After all, the dissolution is associated with complex corrosion processes that alter the surface structure and yield a number of products – that might or might not be hazardous. Depending on the type of magnesium alloy, hydrogen gas can develop during degradation due to insufficient corrosion resistance – to an extent that even a gas cushion is being formed under the patient's skin. Although it is in the surgeon's intention that magnesium screws are degraded by corrosion, during which magnesium oxidizes and hydrogen is produced, the formation of gas cushions should be avoided. If all of a sudden more hydrogen gas is formed than the body can remove, the healing process of the fragile bone can be disturbed.
![]() However, it is precisely this bio-corrosion, to which a magnesium screw is exposed, that has so far been poorly understood. This is where Empa's corrosion researchers come in, using specially developed analytical methods to depict bio- corrosion in the body under conditions that are as realistic as possible. The goal: optimal alloys of magnesium and other biocompatible elements as well as new surface properties for resorbable magnesium screws. Ultimately, the researchers aim for a slow, well-controlled degradation of the implants that does not lead to gas pocket formation within the tissue.
However, it is precisely this bio-corrosion, to which a magnesium screw is exposed, that has so far been poorly understood. This is where Empa's corrosion researchers come in, using specially developed analytical methods to depict bio- corrosion in the body under conditions that are as realistic as possible. The goal: optimal alloys of magnesium and other biocompatible elements as well as new surface properties for resorbable magnesium screws. Ultimately, the researchers aim for a slow, well-controlled degradation of the implants that does not lead to gas pocket formation within the tissue.
"So far, it is already clear that the reaction is different depending on the level of acidity in the tissue," explains Bruin- ink. In a slightly acidic environment, large amounts of hydrogen gas are formed during magnesium corrosion; at a pH value in the alkaline range, carbonate-containing products are produced, among other things, which can even inhibit the desired magnesium degradation. In a neutral environment with a pH value of 7.4, such as in blood, magnesium hydroxides and phosphate products are formed, which at least slow down further corrosion. Blood – as a potent buffer – is able to keep its pH value within a constant range. According to Bruinink, magnesium implants have hitherto been analysed in comparably potent but rather non-physiological buffer systems. He does not consider this procedure to be realistic.
"Blood is a juice of very special kind" – this is how Goethe's restless scholar Faust put it. Whether Doctor Faust knew anything about the so-called interstitial fluid, is not known. The saline fluid with its approximately ten liters far exceeds the volume of blood in the human body. This underestimated "juice" moves slowly between tissues and cells at a speed a hundredfold slower than a snail. And it is precisely this interstitial fluid that is of crucial importance if new implants are to be developed. The healing process of a bone fracture, which is controlled by immune cells and is intended to produce a well-balanced structure of bone resorption and remodelling, is primarily embedded in interstitial fluid.
The acidity of the tissue fluid, however, is subject to much greater variability than that of blood. Depending on the body part and tissue condition, a variety of parameters can affect the inserted screw. In order to provide a realistic prognosis of the course of bio-corrosion in the body, Bruinink has developed experimental analysis techniques and flow cells, in which the pH regulation is modelled on the body. In a battery of ten flow cells, for instance, the researcher inserts samples of magnesium alloys that are washed by artificial interstitial fluid – with the same slowness as in the human body.
In addition to pH measurements, detailed electrochemical characterizations are currently being carried out on the small flow cells. Electrochemical potentials, electrical impedance changes of the interfaces as a feature of corrosion and the generation of hydrogen are evaluated. "The flow cell is a tiny lab that simulates the reality of bio-corrosion," says Bruinink. In the next step, the alloy samples will be brought together with living cells in the minilab in order to imitate events in the body in even greater detail. Bruinink: "As soon as it is clear what actually happens to the magnesium alloys during bio-corrosion, we will be able to produce suitable implants with functionalized surfaces that, for example, promote beneficial reactions of the biological environment."
Magnesium alloys show signs of corrosion when washed with body-like tissue fluid. (black, on the right). If, on the other hand, for the analysis a non-physiological, conventional buffer is used, the magnesium sample remains almost undamaged (white, left). Image: Empa
-
Share

Reproducing historical trombones
Romantic Replicas
To play a piece of music as it was conceived by the composer is a trend. But where can the rare historical instruments be found? The solution would be exact copies of the coveted originals. A team of Empa researchers is analysing such replicas with the aim of reproducing historical trombones with their typical sound. More.

Jean-Marc Wunderli
An Open Ear for Noise
A rippling stream is Jean-Marc Wunderli's favourite sound. However, his everyday research has little to do with calming natural sounds. He deals with completely different ones: noise from aircraft turbines, train noise, busy roads, wind tur- bines and now even drones. All these emissions are part of the research activities of Empa's Acoustics / Noise Control Lab, which Wunderli is heading since last July. More.

Analysis of multi-layered tissues
On Your Medicine's Secret Service
Whether a wound heals well under a dressing cannot be seen from the outside. Empa researchers are now enabling a view through the bandage à la James Bond. The refined application of terahertz radiation could promote the analysis of multi-layered tissues for medical purposes and be used for wound treatment or the diagnostics of blood vessel plaques. More.
