Water-based flow batteries
This is a battery
Non-toxic and scalable water-based flow batteries would be a good solution for storing renewable energy in urban areas – if it weren't for their very low energy density. Empa researcher David Reber wants to remedy the situation with clever materials design.

Two colored liquids bubbling through tubes: Is this what the battery of the future looks like? Empa researcher David Reber has set out to answer this question over the next four years with the support of an Ambizione grant from the Swiss National Science Foundation (SNSF).
So-called redox flow batteries have been known since the 1970s. Unlike conventional lithium-ion batteries, they store energy not in solid electrodes but in tanks containing liquid electrolyte solutions. The charging and discharging process does not take place in the tanks themselves; the electrolytes are pumped through an electrochemical cell instead.
Liquid batteries are impractical for cell phones, laptops or cars. But they are very promising for stationary storage solutions. Since the energy is stored outside the actual cell, flow batteries can benefit from a simple and targeted scaling. A larger electrochemical cell makes the battery charge and discharge faster, larger electrolyte tanks allow it to store more energy.
"As we use more renewable energy, we will need energy storage on a large scale – even in urban areas," Reber says. Another point for flow batteries: If water-based electrolytes are used, they are basically non-flammable, unlike conventional lithium-ion batteries.
Outsourced energy density
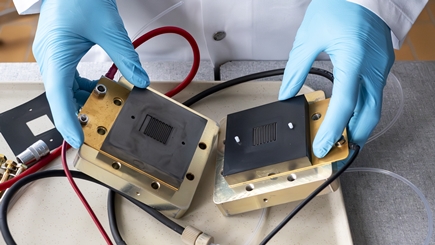
Nevertheless, the technology has not yet caught on. Reber knows the main problem: "Flow batteries have an energy density around ten times lower than batteries made of solid storage materials," he explains. The more storage material can be dissolved in the electrolyte, the higher the energy density of a flow battery. "However, high concentrations thicken the solution, and you need a lot more energy to pump it through the cell," the researcher says.
Reber now wants to solve precisely this problem in his work in Empa's Materials for Energy Conversion laboratory – with an unusual approach. While most projects on flow batteries focus on better solubility of the storage materials, he wants to completely decouple energy storage from the electrolyte solution. "My vision is to develop a hybrid of sorts of a flow battery and a lithium-ion battery," he says. To do this, Reber wants to add solid storage materials, such as those used in cell phone batteries, to the flow battery's tank. "If the dissolved material and the solid storage material are precisely matched, they can transfer energy between each other," Reber elaborates. "This allows the scalability of flow batteries to be combined with the high energy density of batteries with solid storage materials."
Wanted: suitable materials
But first, the researcher needs to find suitable pairs of materials that enable the energy exchange while also remaining stable over an extended period of time. "Ideally, a redox flow battery should be able to operate for about 20 years," he says.
Whether a pair of materials fits together depends on what is known as the redox potential of the substances: at what voltage they donate or accept electrons. "I already have several possible pairs in mind," Reber says. And if a promising pair doesn't quite match, its redox potentials can be manipulated with certain chemical tweaks. One of Reber's ideas is to use a chelate as the dissolved storage material: a multi-armed organic molecule that "wraps" around a metal ion. Depending on how many arms the organic molecule – the ligand – has, the redox potential changes. Reber already conducted research on chelate-based redox flow batteries during his postdoctoral period at the University of Colorado Boulder, for which he will receive the prestigious Battery Division Postdoc Award at the annual meeting of the Electrochemical Society in Gothenburg in October.
At the end of his Ambizione funding period of four years, Reber hopes to have a well-functioning battery with additional solid storage. "If this approach works, the potential applications are very diverse," he says. For example, compact flow batteries with a flexible form factor would be much easier to integrate in urban areas. "All it would take would be pumps and a few pipes," the researcher adds.

Empa Quarterly#81 Battery research
Being able to store energy is a central pillar of a sustainable energy system, since solar and wind energy are not always available in sufficient quantities when they are needed. Good batteries are indispensable for the energy transition, and thus for a more sustainable world. Empa researchers are developing batteries for different applications, from stationary energy storage to electromobility. They are also working on analyzing and recycling end-of-life batteries.
Read the latest EmpaQuarterly online or download the pdf-version.

Empa Quarterly#82 Mining the Atmosphere
To limit climate change, we need to compensate not only for future emissions, but also for historical ones. One solution would be the "atmospheric vacuum cleaner": we remove the excess CO2 from the atmosphere. But what do we do with it? Instead of extracting the carbon for polymers, medicines, fibers, fuels and the like from crude oil, we use atmospheric CO2. This is the simple – yet extremely challenging in technical terms – idea behind Empa's new research initiative, Mining the Atmosphere.
Read the EmpaQuarterly online or download the pdf-version.

Empa Quarterly#83 Perovskites: Versatile cristals
Over 180 years ago, a curious crystal was discovered in the Ural Mountains. Today, it has given rise to an entire class of materials that is of great interest to researchers: perovskites. What all perovskites have in common is their crystal structure, which gives them unusual properties. By changing the exact composition of the perovskite, scientists can control these properties. Empa researchers are using this promising material to develop solar cells, detectors and quantum dots.
Read the EmpaQuarterly online or download the pdf-version.
-
Share






