Nanostructured Materials

We strive to master the whole chain of competence from material designs and systems to their implementa-tion into a device architecture using tailored fabrication and analytical techniques.
Starting from raw materials, we develop and maintain novel synthetic processes including chemical vapor deposition (e.g. graphene), sputter-deposition techniques (e.g. complex magnetic and non-magnetic multi-layer system), particles synthesis (e.g. magnetic particles), surfaces functionalization (e.g.: thiolation, silaniza-tion, peptides and protein grafting) as well as exfoliation and stacking techniques (e.g.. hBN and other 2D ma-terials).
For the materials integration and device fabrication, we pioneer tailored patterning techniques including state-of-the-art nanofabrication, e-beam and optical lithography, ion-beam based patterning, nanoimprint lithogra-phy and printing,
The integrity and functionality of the different material architectures and devices is investigated with state-of-the-art and dedicated, home-built equipment and methods. Such approaches include Raman spectroscopy, Atomic Force Microscopy in UHV and liquid environment, quantitative Magnetic Force Microscopy, failure analysis and X-ray analytical imaging. The latter will integrate the development of X-ray analytical imaging methods allowing a holistic analysis of nano-materials and interfacial strain and defects.
We develop a deeper understanding of the architectures design and properties using modeling (e.g.: multi-scale approaches including DFT and molecular dynamics, machine learning).
The targeted applications are in biochemical sensing (e.g.: biomarkers, proteins), imaging & spectroscopy (e.g. RBCs), and diagnosis (e.g.: protein aggregation diseases) and theranostics (magnetic particles for solid tumors treatment).


Metal-organic architectures for biochemical sensing
Partners: Prof. R. Rossi, Prof. M. Calame (Empa)
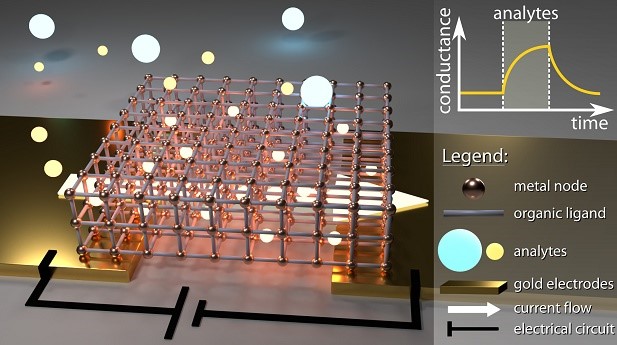
Reference
Conductive Hybrid Cu-HHTP-TCNQ Metal–Organic Frameworks for Chemiresistive Sensing. L. Lüder et al., under review (2021).
Protein Aggregation in Health and Neurodegenerative Diseases
Partners: Dr. Peter Nirmalraj; Dr. Ansgar Felbecker, Kantonsspital St. Gallen; Prof. Dr. Damien Thompson, Univ. of Limerick, Ireland.
Content & Results: Understanding the rules and distinguishing the differences between normal and aberrant protein aggregation is key to answering elemental questions from why we age to what triggers the early on-set of neurodegenerative diseases. Here, we focus on decoding the aggregation pathways of proteins impli-cated in the pathology of Alzheimer's disease using liquid-based scanning probe microscopy, chemical spec-troscopy and machine learning-based data analysis for protein classification in body fluids. To this end, we investigate both protein aggregates at buffered aqueous solution-solid interfaces and in body fluids (blood and cerebrospinal fluid) from individuals at various stages of decline in memory and cognition through a col-laborative project with Kantonal Hospital St. Gallen. We aim to establish that clinically relevant information can be obtained and validated at the nanoscopic scale.
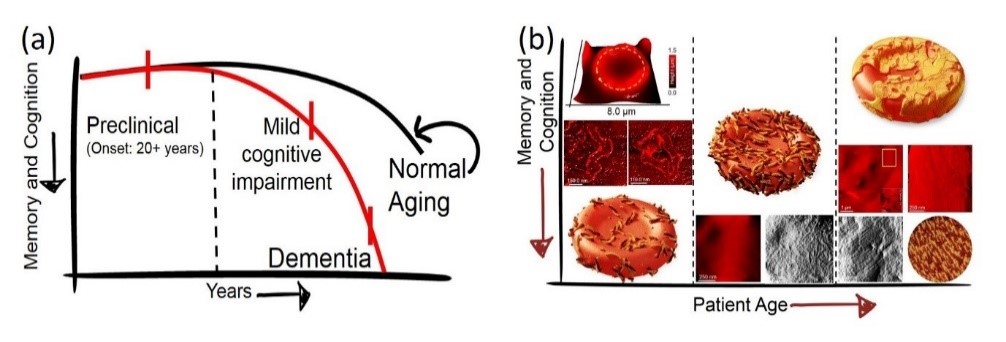
References
Spatial organisation of protein aggregates on RBCs as physical biomarkers of Alzheimer's disease pathology. Peter N. Nirmalraj et al., Science Advances, 7, 39, eabj2137 (2021).
Complete aggregation pathway of amyloid-β (1-40) and (1-42) resolved on an atomically clean interface. Peter N. Nirmalraj et al., Science Advances, 6, 15, eaaz6014 (2020).
Innovative Composite Multilayers for Encapsulation Solutions in Orthopedic and Trauma Surgery
Partner: COAT-X, CSEM, University of Bern
Content: Implantable devices require a high degree of biological safety which includes efficient protection against body fluids and maximal miniaturization. These needs will be addressed using an innovative multilayer encapsulation technology, able to resist acute and chronic inflammatory tissue reactions.
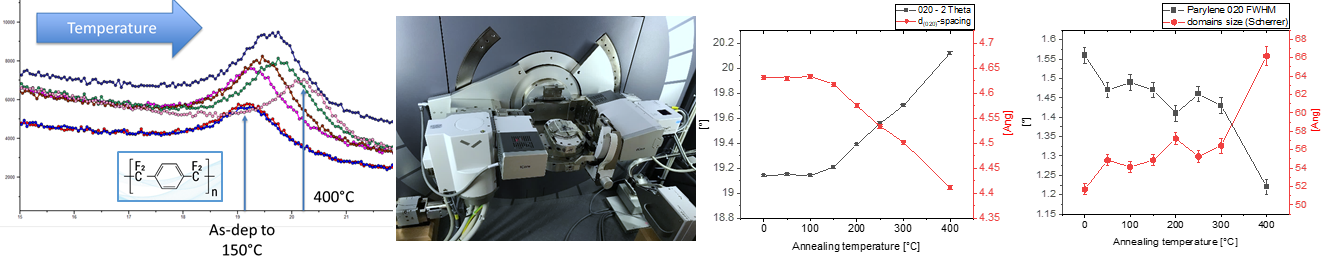
Result: Within the project, the next generation of thin film encapsulation (TFE) is developed to insure a safe and efficient protection of implantable devices. The TFE must resist the inflammatory tissue environment and therefore must be hermetic, thermally stable, have a good adhesion and be mechanically robust. This is ap-proached by a multilayer approach combining polymer- and ceramic-based thin films. The structural parame-ters (layer structure, density, roughness, stability) are understood via non-destructive XRD and SAXS methods and finally integrated into the design of the TFEs.
Thin Film Magnetic Nanoparticles for Theranostics
Partners: Prof. Dr. Inge K. Herrmann (ETHZ & Empa), Dr. Peter Wick, Prof. Dr. Hans J. Hug (Empa). Prof. Dr. Dieter Suess (University of Vienna). Industrial Partners for high-throughput sputter-deposition and microfabri-cation.
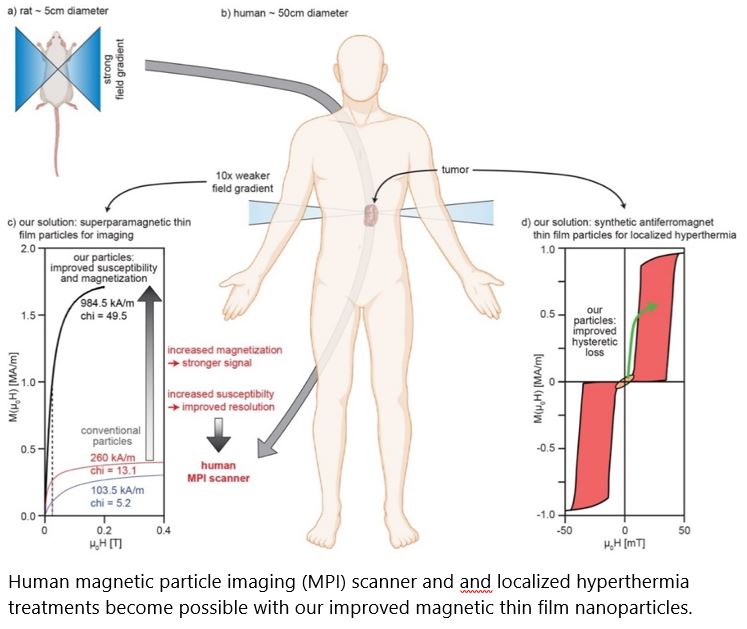
Results: Sputter-deposited of magnetic thin film systems are used for wide-spread applications such as cloud-based data storage, sensors e.g. for automotive applications. First steps have been undertaken to har-ness this technology for MNPs with enhanced properties for biomedical applications. However, to date, the current system designs have not yet optimally addressed the biomedical needs. We have developed two can-didate systems [superparamagnetic (SP-MDPs) and synthetic antiferromagnetic (SAF-MDPs) particles, 1 patent appl. EP20211045 by Profs. Hug/Suess / 1 in preparation] overcoming the current limits. With our competen-cies spanning from thin film magnetism, micromagnetic simulations, microfabrication, to pre-clinical MNP de-velopments, two partners with established expertise in upscaling, fabrication and clinical opinion leaders, we can overcome current limits and demonstrate the advantageous properties arising from thin film-based MNPs for the envisioned biomedical applications.
Reference
Synthetic Antiferromagnet Disk-Shaped Particle and Suspension comprising such Particles. H.J. Hug and D. Suess, Patent application EP20211045, submitted Dec. 2020.
Partners: Prof. Dr. K. Maniura, Dr. M. Rottmar (Empa)
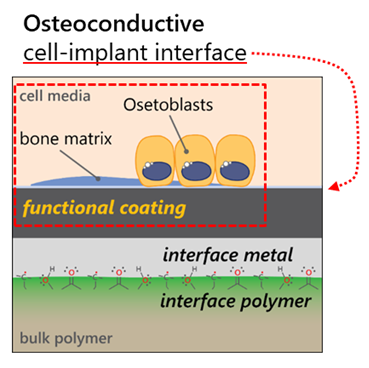
The aim of the project is twofold: First, we look to gain a fundamental understanding of the metal-polymer interaction by studying the effects of the processing stages (surface plasma activation, and the metallization itself) on the metal-polymer interphase structure and subsequent coating adhesion. Specifically, we examine the surface and near-surface chemistry resulting from the two processing stages, using a variety of surface analytic methods. We further probe the role that coating energetics plays on interphase and adhesion, span-ning the energetics range of off-axis sputtering to High Power Impulse Magnetron Sputtering (HiPIMS). The outcome of this phase of the project is to design a metal-polymer interphase that leads to strong coating ad-hesion.
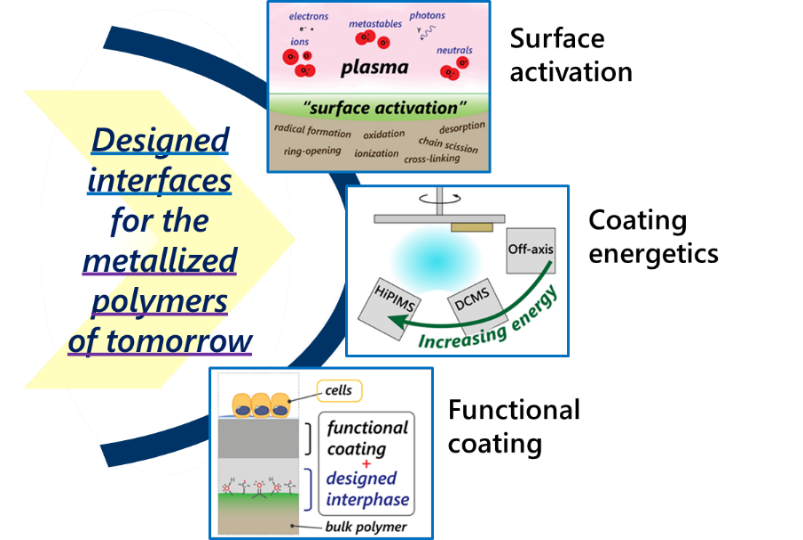
In this way, the project produces a coated polymer solution for enhanced bioactivity, in addition to a funda-mental understanding of the metallization process as it relates to coating adhesion. Future projects will build on the knowledge and technology developed here. These will serve as a base to integrate further device func-tionalities such as sensing, energy harvesting and remote actuation.
Reference
Hierachically structure polydimethyloxane films for ultrasoft neural interfaces. B. Osmani et al., Micro and Nanoengineering 7 (2020) 100051.
Further selected references
A. K. Maurya, A Parrilli, T Kochetkova, J Schwiedrzik, A Dommann, A Neels (2021). Multiscale and multimodal X-ray analysis: quantifying phase orientation and morphology of mineralized turkey leg tendons. Acta Bio-materialia, 169–177. https://doi.org/10.1016/j.actbio.2021.05.022
Netkueakul W, Korejwo D, Hammer T, Chortarea S, Rupper P, Braun O, Calame M., Rothen-Rutishauser B, Buerki-Thurnherr T, Wick P and Wang J (2020). Release of graphene-related materials from epoxy-based composites: characterization, quantification and hazard assessment in vitro. Nanoscale 12, 10703-10722. https://doi.org/10.1039/C9NR10245K
O. Synhaivska, Y. Mermoud, M. Baghernejad, I. Alshanski, M. Hurevich, S. Yitzchaik, M. Wipf & M. Calame (2019). Detection of Cu2+ Ions with GGH Peptide Realized with Si-Nanoribbon ISFET, Sensors 19(18), 4022. https://doi.org/10.3390/s19184022
